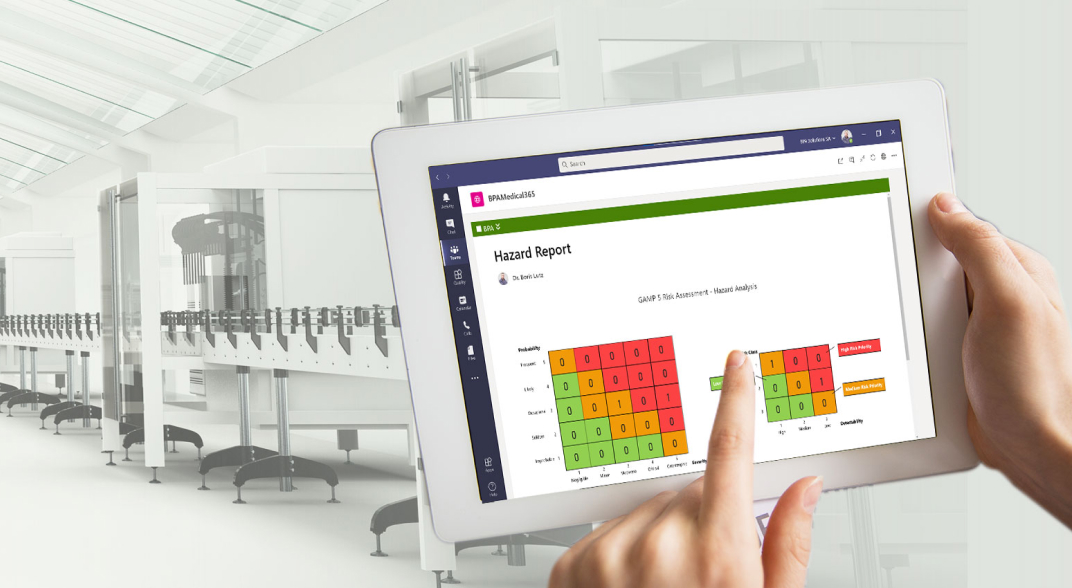
Simplify Compliance to Medical Regulations
The need to comply with the international standard ISO 13485 requirements, Medical Device Regulations (MDR) and FDA is challenging for managers in medical device-related industries, healthcare, pharma and life science sectors.
BPAMedical365® is an innovative QMS software for medical quality & compliance management, ready to use in your trusted Microsoft 365 environment, and simplifying compliance to medical regulations. The software includes all solutions, features and benefits of BPAQuality365® plus additional eSignature features and product lifecycle management.
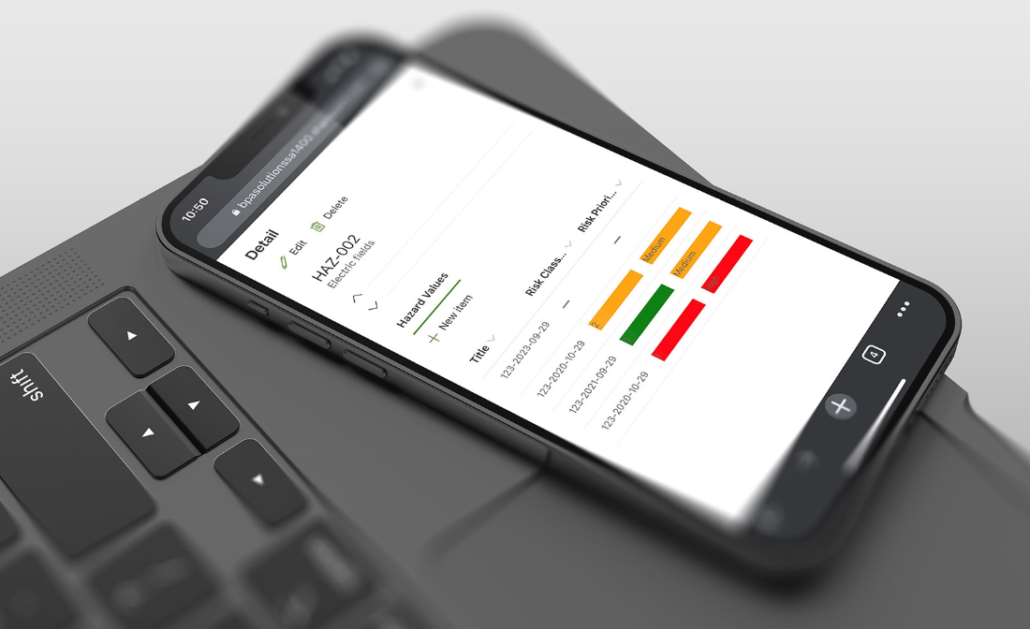
Medical Hazard Software on Phone
Request a FREE Trial Access
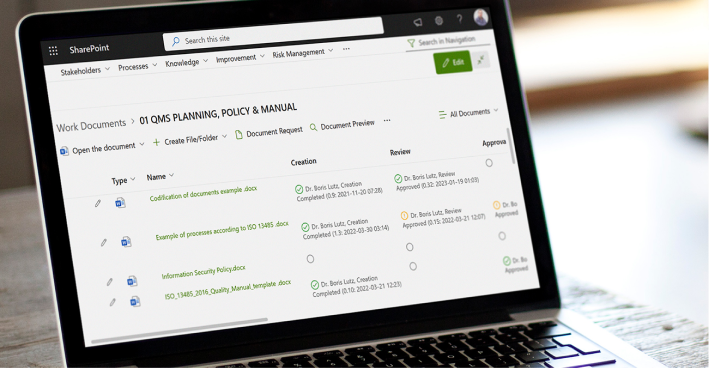
Streamline Document Management
Document management is highly regulated in the medical industry. We have developed specific tools and automated workflows to comply with medical regulations. The BPA eSignature component included in our medical QMS software makes sure only authorized people can sign by using the trusted Microsoft multi-factor authentication system. Automated workflows reduce manual work and streamline complex document management processes.
Satisfy GxP Guidelines
BPA Medical GxP Software
Ensure Valid Approvals with eSignatures
The BPA eSignature component provides a simple way for everyone to sign documents or forms based on the robust Microsoft Multi-Factor Authentication system, used by most companies with Microsoft 365. eSignatures are used to make sure the right persons have reviewed and approved important procedures, technical files, or records in compliance with regulations.
Satisfy GxP Guidelines
BPA’s medical QMS software and Microsoft Office 365 include solid features to satisfy GxP guidelines and 21 CFR Part 11 requirements for electronic records, like document retention, access control, and audit trail. BPA’s eSignature module gives the extra security required when approving items. Our eSignature module gives the extra security required when approving items.
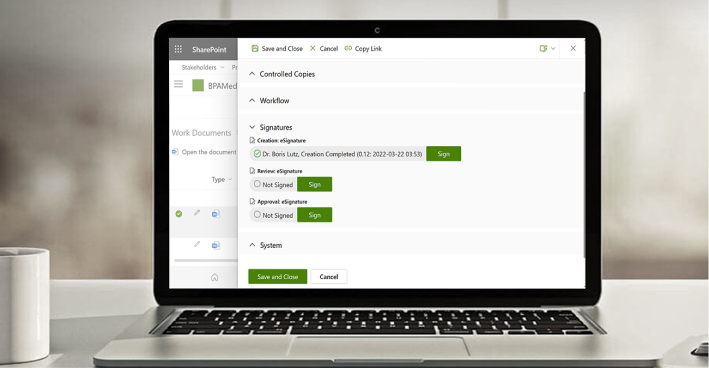
Satisfy GxP Guidelines
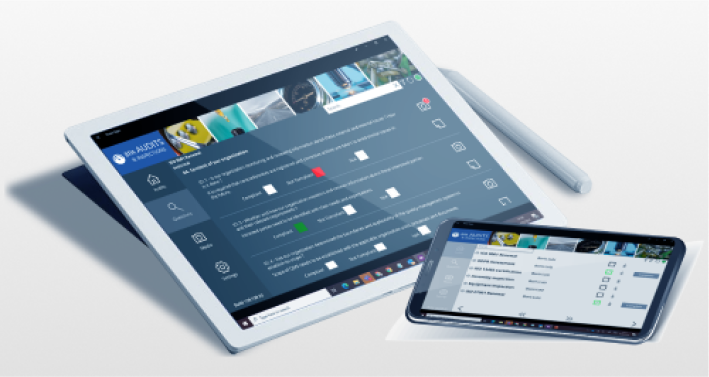
BPA Medical GxP Software
Do More with Power Extensions
The software provides a robust collaborative platform for the whole organization, leveraging SharePoint and Teams capabilities for instant discussions and video conferencing. Go a step further and meet Industry 4.0 objectives with the Audit Power Apps, Incident and Failure Catalogue Power Apps, productive chatting, AI and prebuilt automated workflows.
Validate the Software
Our software validation quick start package simplifies software (SW) validation, and includes document templates, like a SW validation plan, SW validation SOP, requirement specifications, and test case samples for BPAMedical365®. For a turnkey project, our partner offer all needed software validation services.
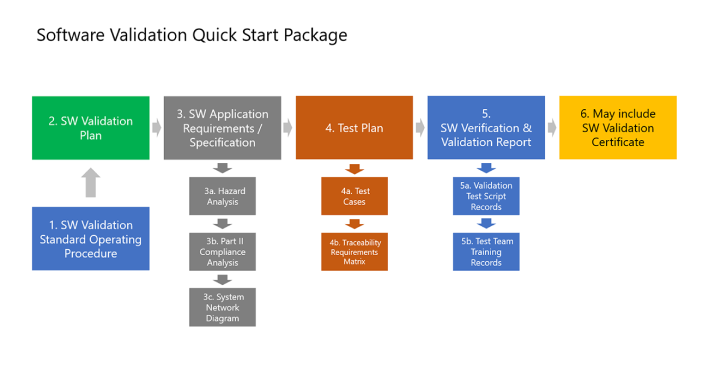
Validate The Software
Instantly find out more!

Engaging
Boosting user experience & have fun!

Agile
Enhancing your existing assets & be unique!

Innovative
Enabling modern technologies & go smart!
Microsoft 365 is the Right Technology For Your QMS

Trust
M365 is trusted by enterprises worldwide.

Security
M365 provides the best security for your QMS data.

Lower Cost
Lower cost in using acquired M365 tools for your QMS
What’s New
Unlock Intelligent Quality: insights from our webinar
This month we had our second Webinar uncovering how BPA Pilot, our newly...

Go a step further with Gen AI in your eQMS: join BPA’s 3-Month Pilot Program!
Are you ready to take your QMS to the next level? BPA Solutions is proud to...

How Generative AI leverages your internal eQMS data
GenAI, or Generative Artificial Intelligence, brings substantial benefits to...