Medical Device Quality Management Software for Small Businesses

Quality management software for medical device manufacturers is usually complex, expensive and out of reach for small and medium companies.
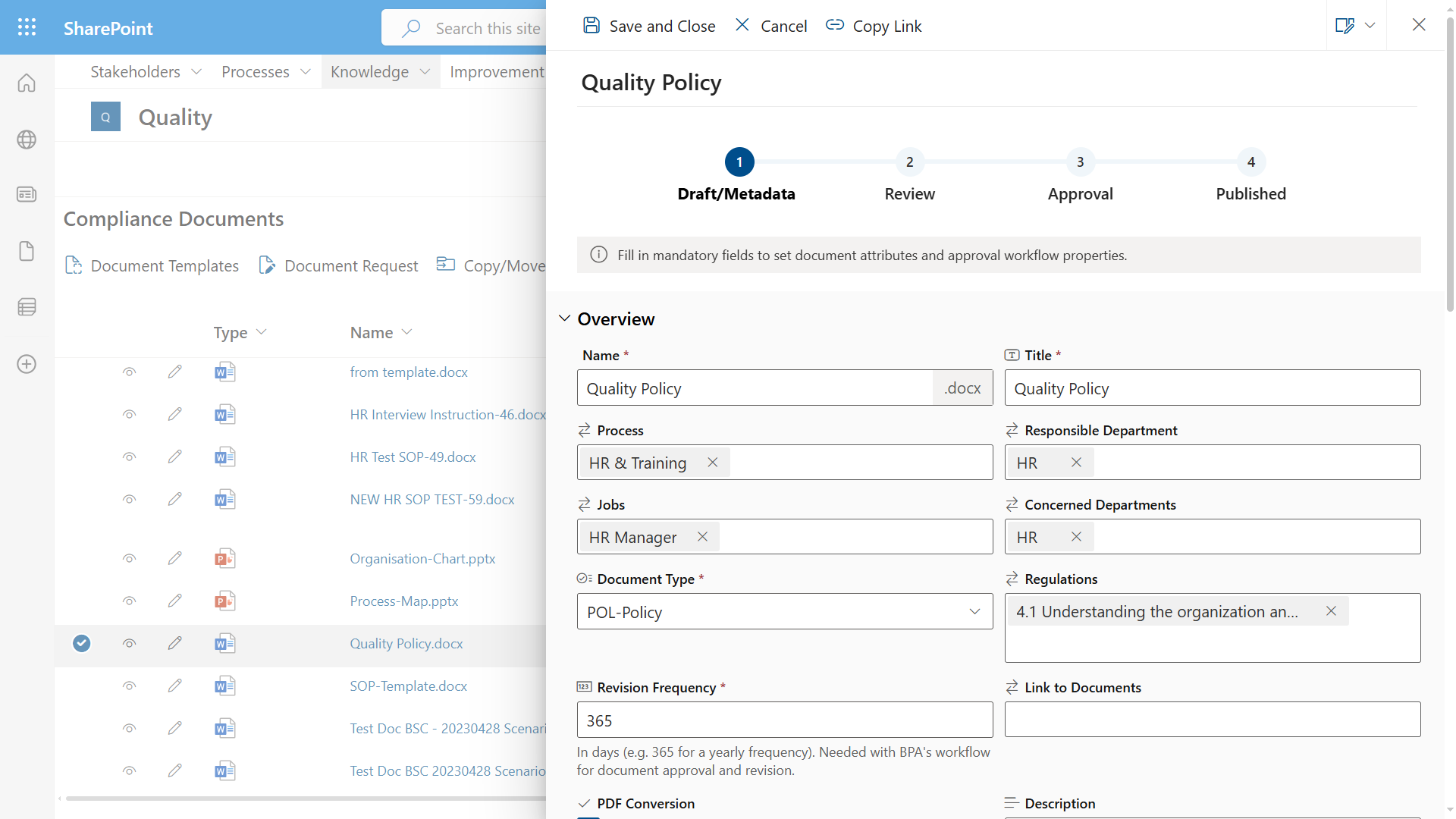
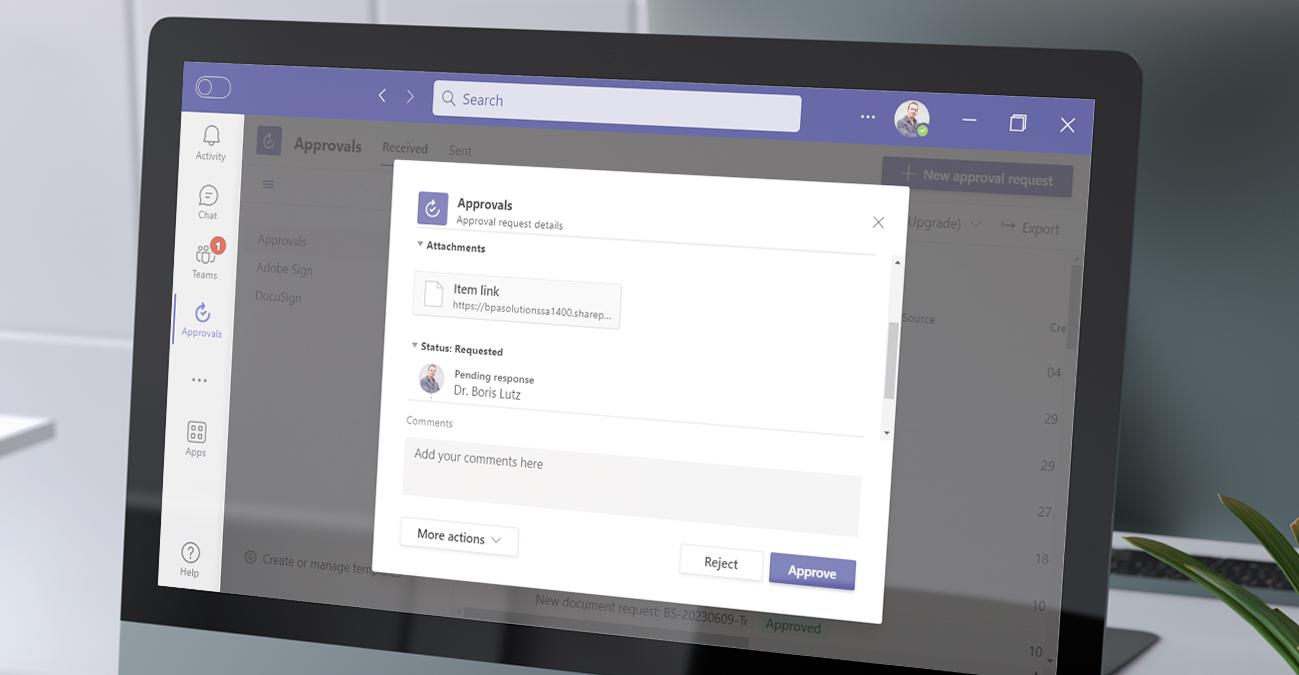
However this is no longer the case, BPA has just released an integrated quality and risk management software, affordable, flexible and adapted to small and mid-sized medical device companies. A new digital signature module has been developed to comply with 21 Part 11 CFR regulations (view video here).

Built on the CFR 21-compatible Microsoft Office 365 technology, BPA Quality and Risk Management is a cloud-enabled, secure, and scalable solution, delivering superior document management, workflow, collaboration and reporting tools in one single interface and providing an unequaled user experience.
No need for cumbersome and costly paper systems anymore, our digital QMS software facilitates communication between siloed departments, increases efficiency with automated processes and saves cost.
Office 365 + BPA Quality is the ideal QMS solution for small and mid-sized medical device companies.