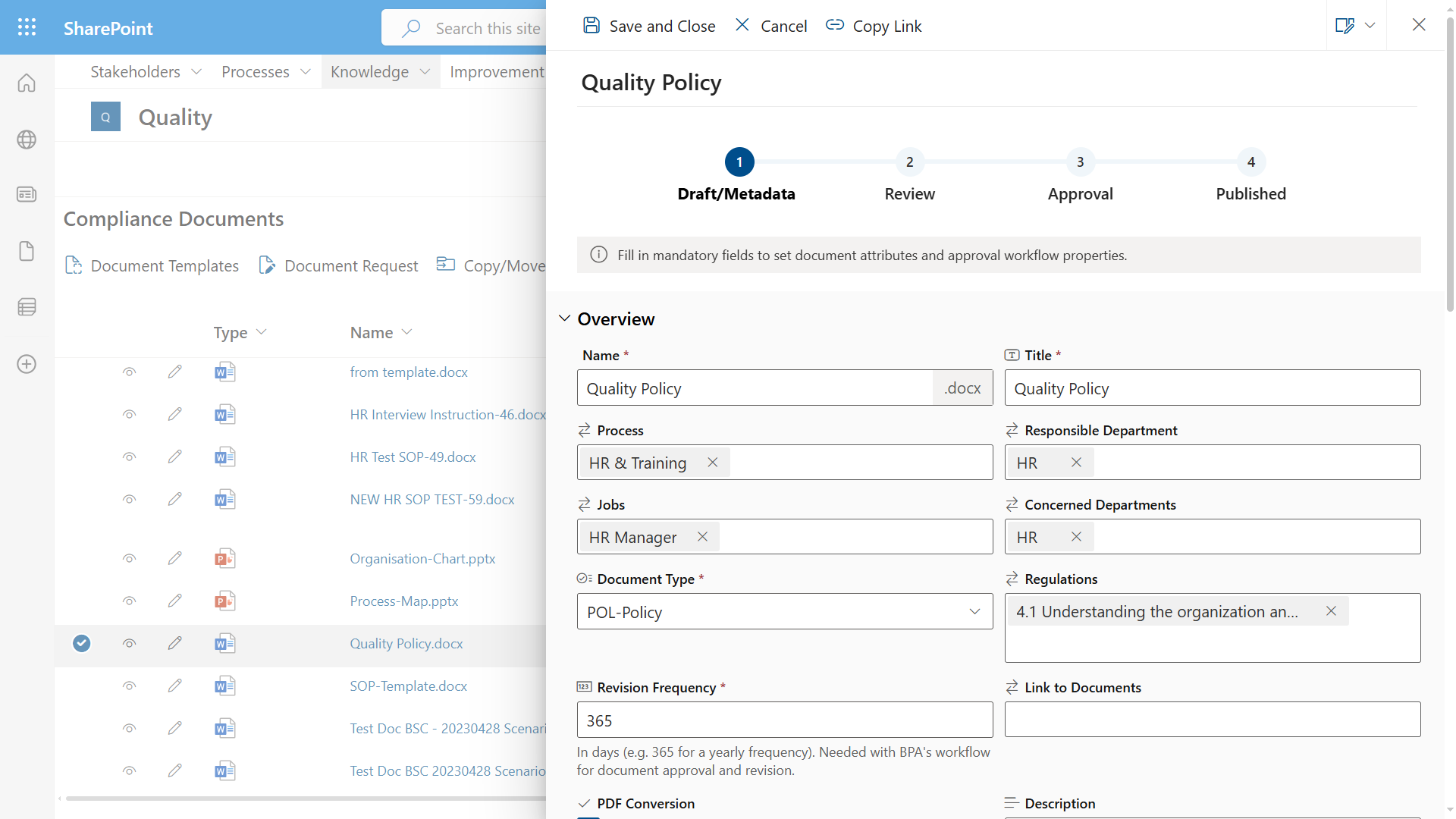
Compliance Document Management for the Medical Industry

Compliance document management is highly regulated in the medical industry. BPA has developed specific tools and automated workflows to comply with medical regulations, like ISO 13485, FDA part 11, MDR and others.
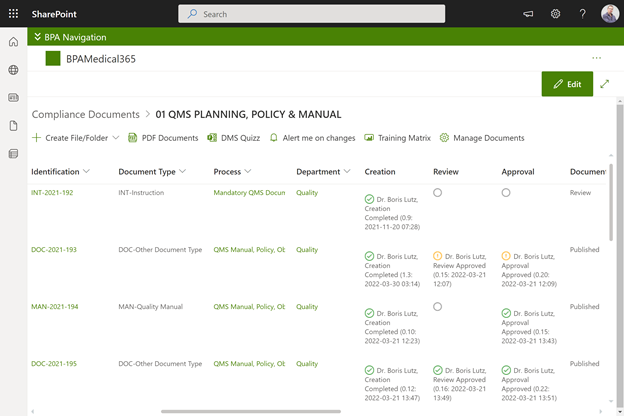
Document preparation area with approval eSignatures in BPAMedical365.
We designed different areas in BPAMedical365 to prepare and approve documents, publish approved documents and train collaborators, and to publish effective documents in PDF format.
The BPA eSignature component makes sure only authorized people sign by using Microsoft multi-factor authentication with face recognition or other procedures.
eSignatures will show as invalidated when the protected document content is changed and selected users will be notified.
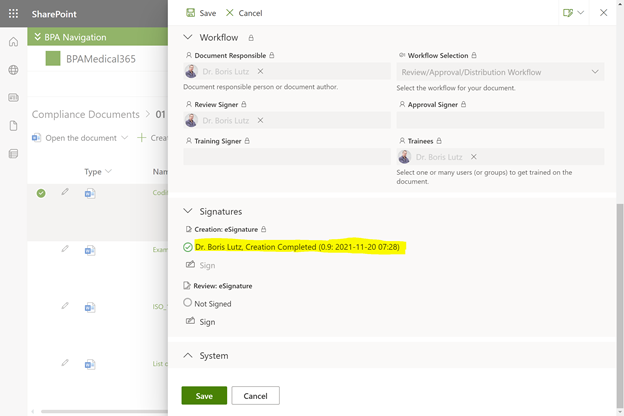
BPA eSignature field in the document properties form.
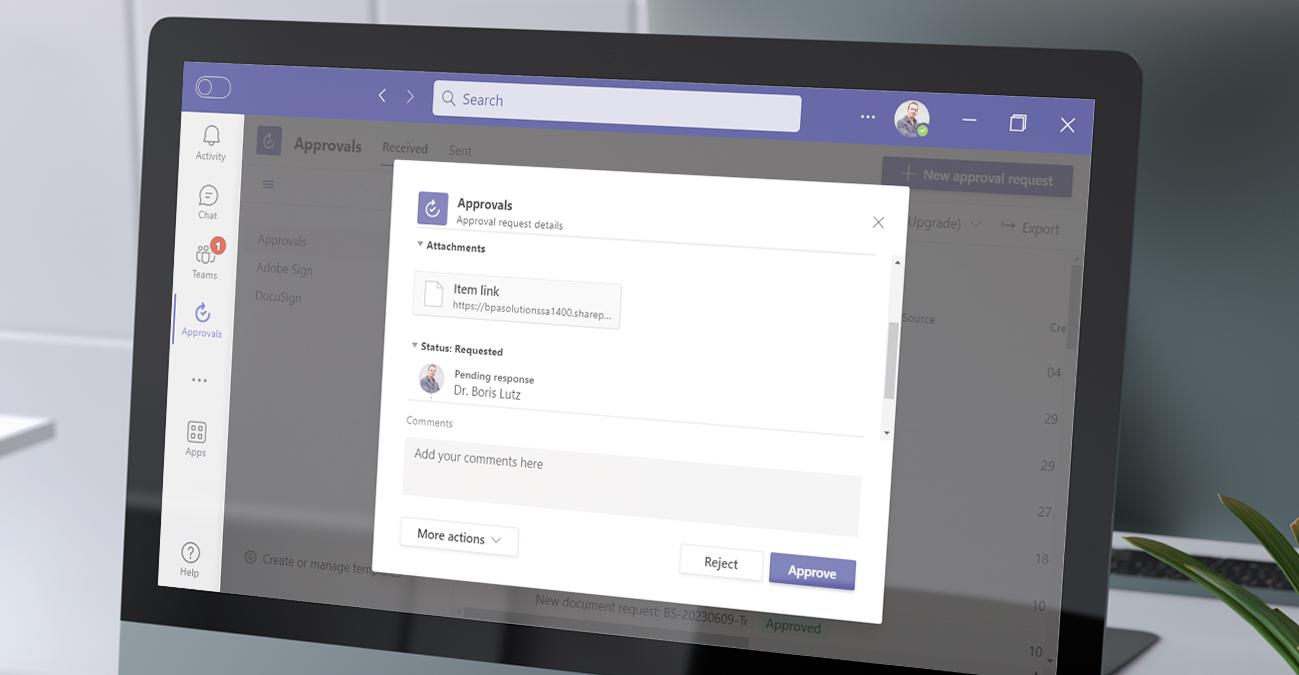
We developed prebuilt automated workflows for document approval and publication. Approval workflows ensure documents are signed by authorized users in the different steps, and prevent the same signer to sign multiple steps.
Trainees will be invited to in-class document training sessions. Collaborator knowledge on documents can be tested with simple Form quizzes. At the end of the process, documents are converted to PDF and published as effective.
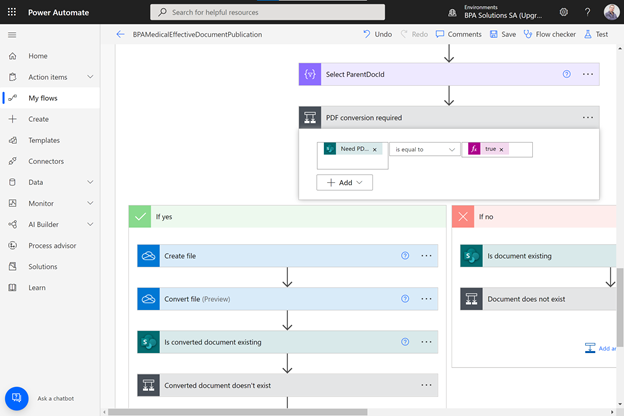
Prebuilt automated workflow for document publication on Power Automate.
Together with Microsoft 365 technologies, BPAMedical365 simplifies your journey to be compliant with medical regulations.