BPA eQMS software solutions benefit companies in the pharmaceutical sector by supporting quality management, document control, compliance management, risk management, training and competency management, and change control processes. By leveraging these software solutions, pharmaceutical organizations can improve operational efficiency, ensure regulatory compliance, mitigate risks, and enhance patient safety and product quality.
Quality management
BPA software helps pharmaceutical companies effectively manage their quality management processes. It allows organizations to create and enforce quality standards, track deviations and non-conformances, and implement corrective actions. The software ensures compliance with regulatory requirements, reduces the risk of product recalls or regulatory penalties, and improves overall product quality and patient safety.
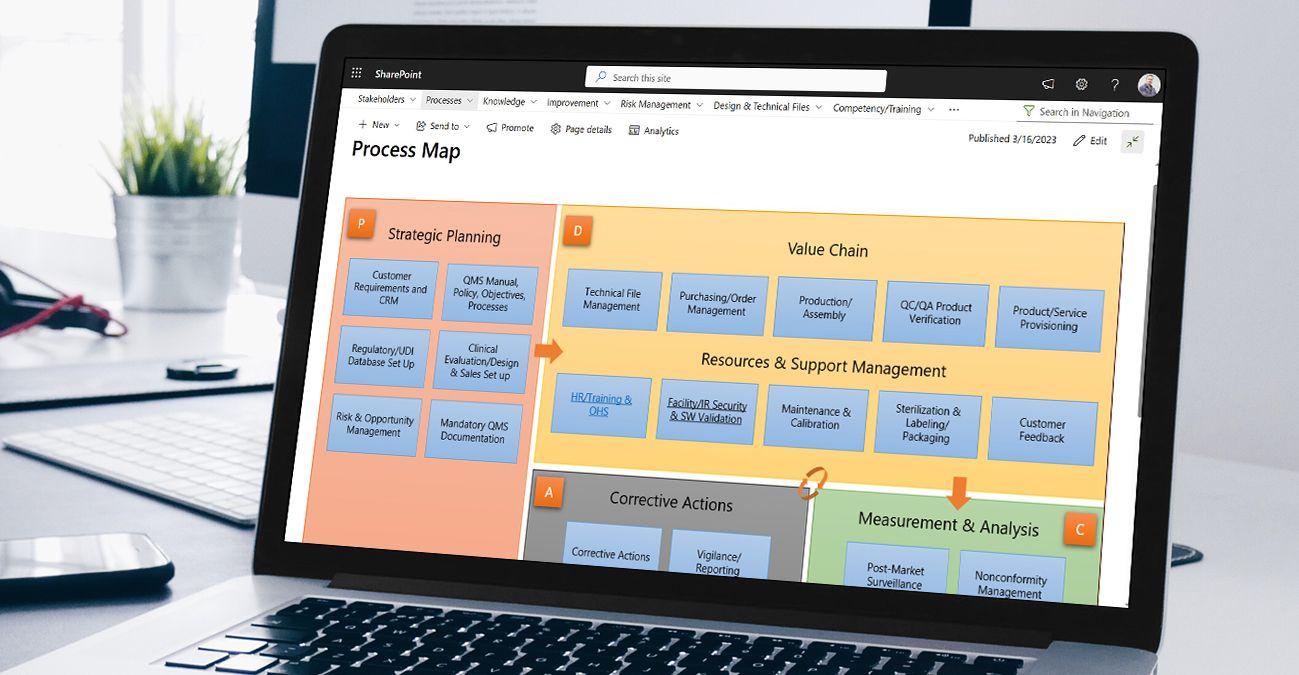
Document control
BPA eQMS software facilitates efficient document control for pharmaceutical organizations. It provides a centralized repository for storing and managing critical documents such as standard operating procedures (SOPs), protocols, and technical files. The software enables version control, document approval workflows including e-signatures, and access permissions, ensuring that employees have access to the most up-to-date and accurate information.
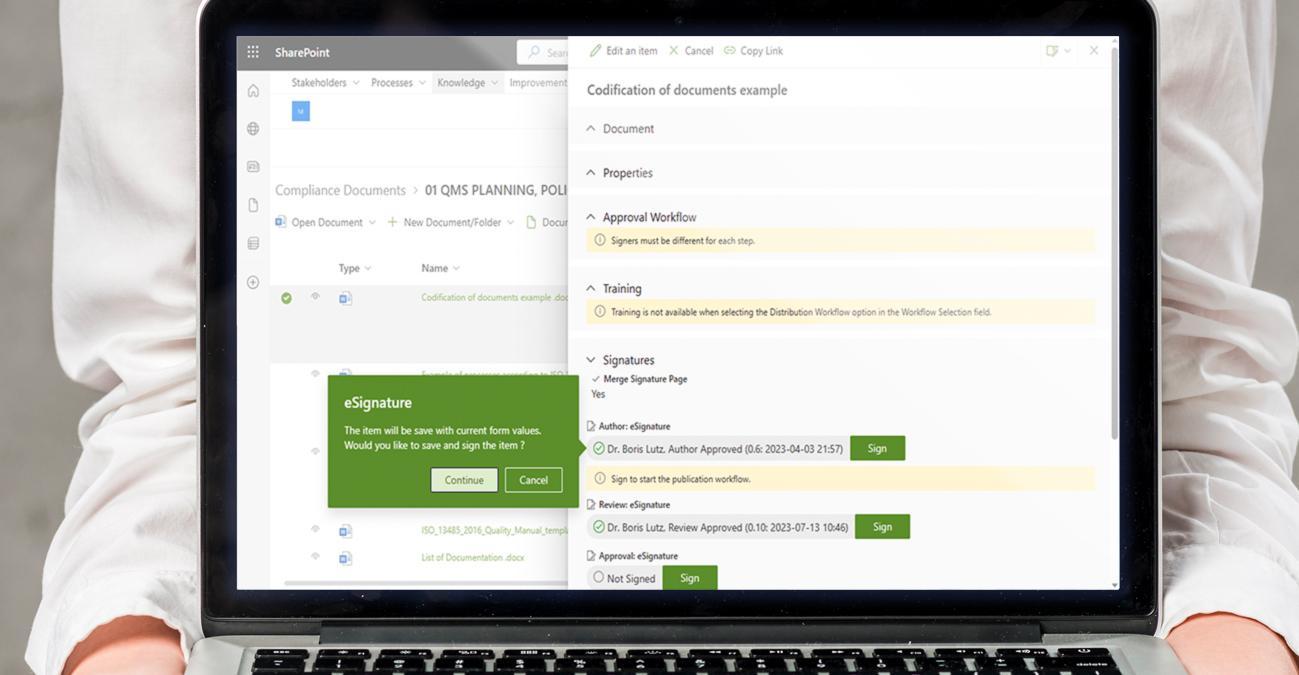
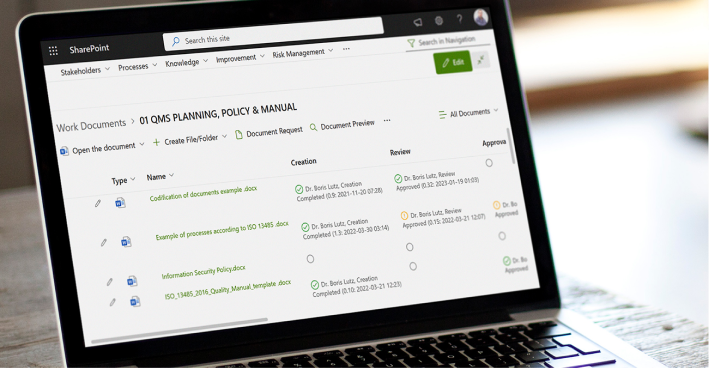
Compliance management
BPA software supports pharmaceutical companies in adhering to strict regulatory requirements. It helps document and manage compliance processes, including regulatory audits and inspections. The software assists in maintaining compliance with industry standards and regulations such as Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP). This ensures regulatory compliance, reduces risk, and strengthens the company’s reputation.
Risk management
BPA software solutions enable pharmaceutical organizations to identify, assess, and mitigate risks associated with their products and processes. It provides tools for documenting and analyzing risks, implementing risk control measures, and monitoring risk mitigation actions. The software helps companies proactively manage risks, minimize potential harm to patients, and maintain product safety and efficacy.
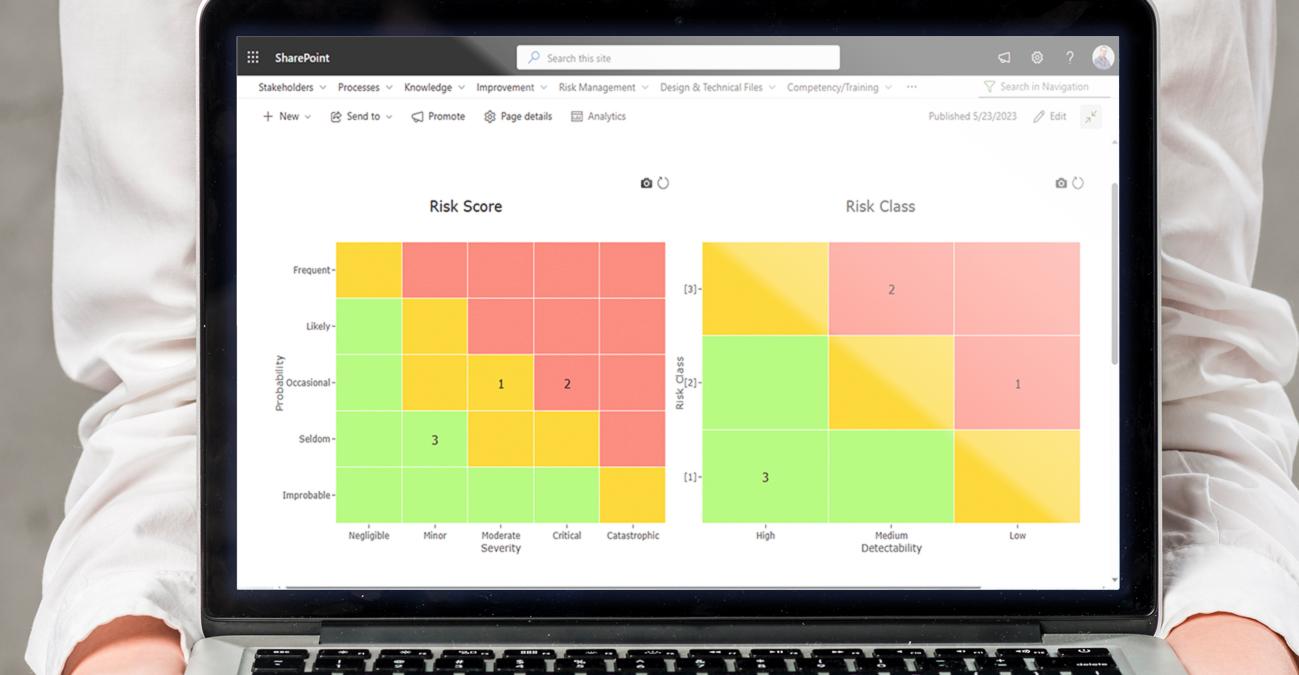

Training and competency management
BPA software supports training and competency management in the pharmaceutical industry. It allows organizations to create and deliver training programs, track employee training records and certifications, and evaluate employee competency. The software ensures that employees are adequately trained and competent to perform their roles, reducing the risk of errors and ensuring compliance with training requirements.
Change control
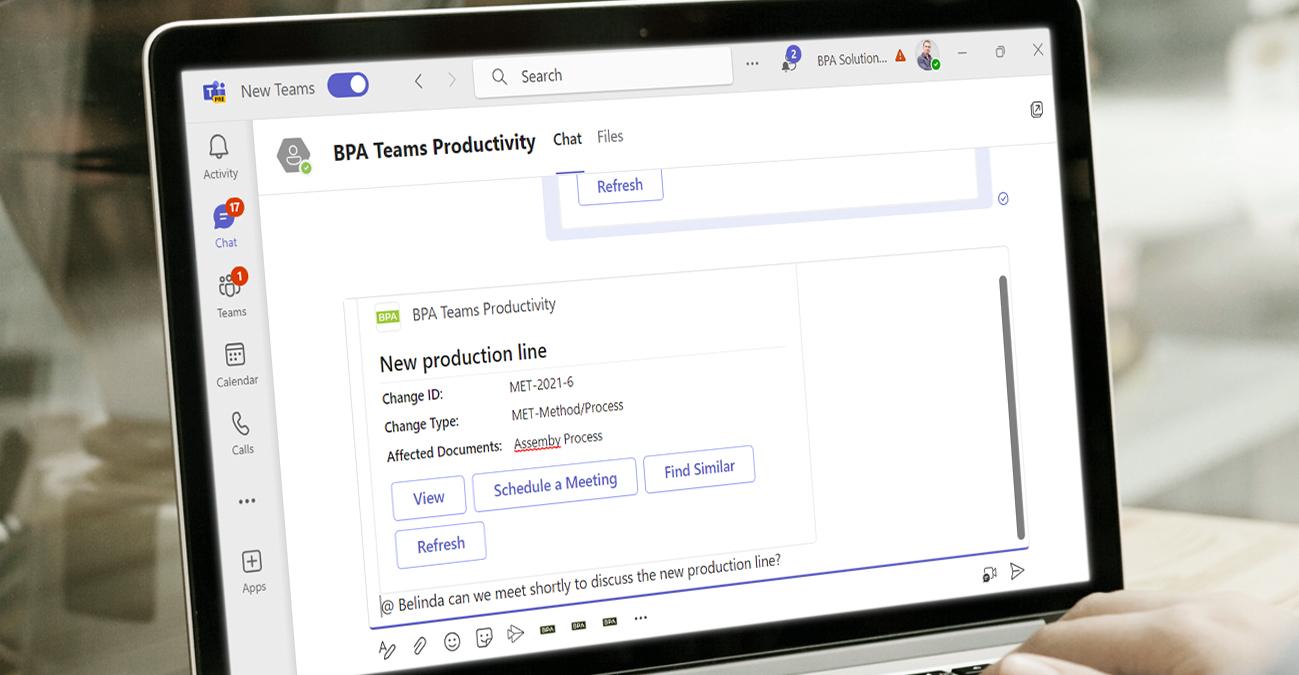
BPA eQMS software facilitates effective change control in the pharmaceutical sector. It provides a structured and documented approach to managing changes in processes, systems, or products. The software helps evaluate change requests, assess potential impacts, implement changes, and track their effectiveness. This ensures that changes are properly managed, validated, and documented, minimizing disruptions and maintaining product quality and safety.

Engaging
Boosting user experience & have fun!

Agile
Enhancing your existing assets & be unique!

Innovative
Enabling modern technologies & go smart!
Microsoft 365 is the Right Technology For Your QMS

Trust
M365 is trusted by enterprises worldwide.

Security
M365 provides the best security for your QMS data.

Lower Cost
Lower cost in using acquired M365 tools for your QMS
What’s New

Shift to ultimate quality management with BPA Premium edition
We are proud to announce the next-coming release of our eQMS Premium Edition...

Reshape audit management with BPA and GenAI
Auditing the QMS is essential to ensure...
Unlock Intelligent Quality: insights from our webinar
This month we had our second Webinar uncovering how BPA Pilot, our newly...


